What is energy coupling? How does it actually work? Here is a full guide of everything you need to know about energy coupling.
Guide to Energy Coupling: What is It and How It Works
Chemical reactions can take various forms. While some of these reactions require energy to take place, others, on the other hand, produce energy. For example, a catabolism chemical reaction is the one that produces energy, while an anabolism reaction is the one that requires energy.
So, what is energy coupling? In this article, we are going to explore deeper into what energy coupling entails, as well as how it works.
But, first things first. Let’s first look at the energy coupling definition.
What is Energy Coupling?
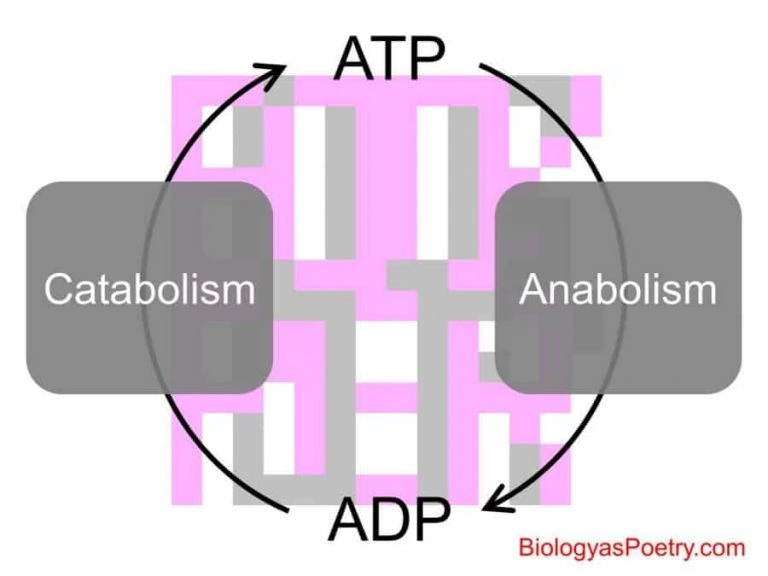
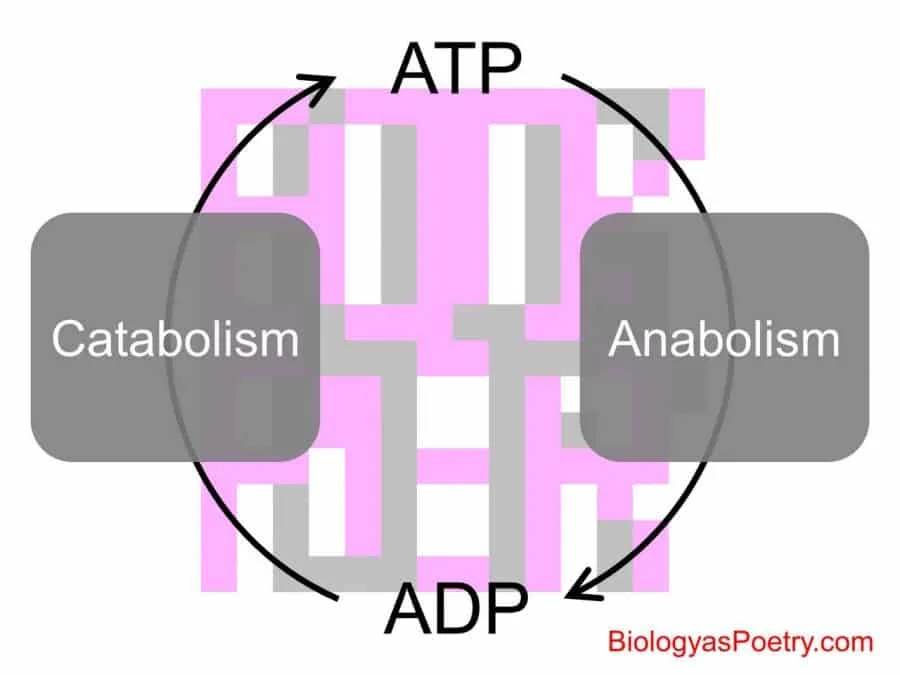
When we talk of energy coupling, it refers to the process of energy transfer, from a catabolism reaction to an anabolism chemical reaction. It simply refers to the process of using an exergonic process to facilitate an endergonic process.
This means that the energy an exergonic process release is used to enable an endergonic process. In this process, ATP is necessary. The ATP acts as the energy currency for the energy coupling process.
In essence, the ATP is used in various chemical reactions that need energy, as a booster for these reactions.
In organisms, energy coupling is typically shown based on ATP production and hydrolysis. Catabolic reactions generate the ATP, while the ATP produced, drives forward the anabolic reactions.
In the electronics field, energy coupling refers to either a desirable or an undesirable energy transmission from one medium, to another — for example, energy transfer from an optic fiber or a metallic cable, to a different medium. The coupling can also refer to the transmission of electrical energy to a different circuit segment from another segment.
So, What is the Role of the ATP In This Process?
Before we get into which role the ATP plays in energy coupling, let’s understand the meaning of some terms in this topic.
- Endergonic reaction: The term is used to describe a chemical reaction that takes in energy (heat) from the environment.
- Exergonic reaction: This describes a reaction that generates or releases energy into the environment.
- Gibbs free energy: This is the amount of maximum effort available, resulting from a system under constant pressure and temperature.
- Hydrolysis: This is a chemical decomposition process which involves the bond splitting through water addition.
- ATP (Adenosine triphosphate): A chemical compound (organic) used to provide energy that drives numerous processes in living organisms’ cells. These include things like nerve impulse propagation, muscle contractions, chemical synthesis, and more.
How Does ATP Play a Role In Energy Coupling

Well, ATP in cellular processes is typically regarded as the energy currency. It offers the energy necessary for both endergonic (energy-consuming) reactions, and exergonic (energy-generating) reactions, which need a small energy input for activation.
The energy necessary for these reactions is generated when a reaction breaks the chemical bonds in the ATP. The energy generated from the reaction can be used to drive cellular processes. Its good to note that, the more the bonds present in a molecule, the higher the energy potential it has.
And, since these bonds in the ATP are easy to break and transform, ATP acts much like a battery (rechargeable one) to power various cellular processes right from protein synthesis to DNA replication.
One important thing to note, though, is that the ATP molecule is highly unstable. Therefore, it should be put to work as fast as possible least it dissociates. The ATP molecule naturally disassociates to form ADP + Pi, releasing the free energy in the process as heat.
The process, through which the energy within these bonds of the ATP is harnessed, is what we refer to as energy coupling. This means that ATP is the driving force in energy coupling.
But, how much energy (free energy) is naturally generated from the ATP hydrolysis process? And, how much of this energy is useful for cellular work?
Well, the hydrolysis of precisely one mole of an ATP molecule, calculated as ∆G (free energy) is −7.3 kcal/mole (−30.5 kJ/mole). This is only possible under standard conditions.
On the other hand, the ∆G (free energy) for hydrolysis in a live cell almost doubles the amount at standard settings. That is 14 kcal/mole (−57 kJ/mole).
How Energy Coupling Works
Sodium-Potassium Pumps

The sodium-potassium pumps can illustrate an excellent example of energy coupling. Here, cells combine an exergonic reaction (ATP hydrolysis) with an endergonic reaction of a cellular process.
For instance, the transmembrane ion pumps present in nerve cells, pump ions through cell membranes, to generate an action potential, using the free energy from ATP. A sodium-potassium pump pushes out sodium (Na) from a cell, and potassium (K) into a cell.
Hydrolysing the ATP molecule helps to transfer its gamma phosphate, through the phosphorylation process, into the protein pump. The sodium-potassium pump receives the ∆G (free energy), which enables it to undergo a conformational change releasing three sodium ions to the exterior of the cell.
Two extracellular potassium ions (K+), which are bound to the protein, causes a change in the shape of the protein, and the discharge of the phosphate. When free energy is donated to the sodium-potassium pump, an endergonic reaction takes place.
Energy Coupling and Metabolism
In cellular metabolism, or nutrient’s synthesis and breakdown, specific molecules must be slightly transformed to become substrates necessary for upcoming steps in the series of reactions.
During the very initial cellular respiration steps, glycolysis (breaking down of glucose) takes place. Here, ATP is necessary for glucose phosphorylation process, which creates an unstable but high-energy intermediate.
The phosphorylation reaction brings about a transformational change through which the “phosphorylated glucose molecule” is converted into “phosphorylated sugar fructose” using enzymes.
This fructose is an important intermediate for the glycolysis process to happen. Here, the ATP hydrolysis, which is an exergonic reaction, is coupled with an endergonic reaction (conversion of glucose) to be used in metabolism.
Importance of Energy Coupling
The hydrolysis process of any ATP molecule facilitates in the breakdown of high-energy bonds (phosphate bonds). In the process, high measures of energy are released in an exergonic form. The coupling process helps to convert the energy generated into an endergonic form, ensuring that the energy is not lost as heat.
Coupling often occurs through a mutual intermediate. This means that the end product of a certain reaction is received and used in another reaction as the reactant.
When the coupling process involves an ATP molecule, the common intermediate is, in most cases, a phosphorylated molecule. A good example of how the process work is in the creation of sucrose from fructose and glucose.
In this case, the formation of sucrose requires an input of energy: its ΔG is about +27kJ/mol within standard conditions. On the other hand, an ATP hydrolysis produces around -30kJ/mol within the standard settings.
What this means is that the energy the process generates is enough to cater to the energy requirements in the sucrose molecule synthesis.
There are normally two reactions here, including:
- The formation of an intermediate (phosphorylated glucose) through an energy-consuming reaction.
- A reaction between the glucose intermediate and fructose to produce sucrose is the second one. Since glucose-P is quite unstable, this reaction is spontaneous and generates energy.
Conclusion on Energy Coupling
Some reactions occur and release energy, such as the hydrolysis of an ATP molecule. On the other hand, some other reactions require some energy to happen.
Energy coupling is necessary to ensure that the energy generated in the first reaction does not go to waste as heat.
Instead, it can be used as fuel for the second reaction that requires energy.
Related Resources
- Everything You Need To Know About Flora And Fauna
- 19+ Energy Conservation Methods: Eco-Friendly Ways to Reduce Energy
- A Definitive Guide to Building Energy Management Systems
Green Coast is a renewable energy and green living community focused on helping others live a better, more sustainable life.
We believe that energy and green living has become far too complex, so we created a number of different guides to build a sustainable foundation for our future.